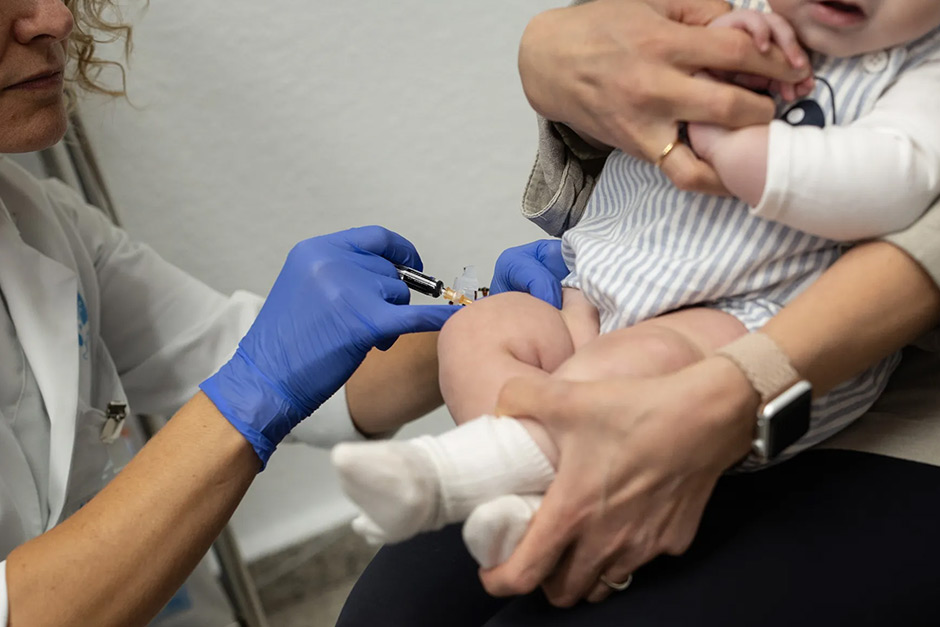
The Centers for Disease Control and Prevention (CDC) recently made two significant announcements regarding childhood vaccines, showcasing their dual role: embracing critical advancements while maintaining a firm commitment to rigorous safety and efficacy standards. In moves that will undoubtedly shape pediatric care across the nation, the agency approved a crucial update to protect the youngest patients, even as it decisively rejected a new, more contentious vaccine proposal.
A Game-Changing Shield for Our Littlest Ones
The first, and widely celebrated, decision involves a major stride in protecting infants from Respiratory Syncytial Virus (RSV). RSV is a common respiratory virus that can cause severe illness, particularly in newborns and young infants, often leading to hospitalizations. The CDC has officially recommended a new protective measure – a long-acting monoclonal antibody called nirsevimab – for all infants younger than 8 months born during or entering their first RSV season. Additionally, a select group of older infants and toddlers at increased risk for severe RSV disease can also benefit.
This recommendation marks a significant shift in how we approach RSV prevention. Previously, preventative options were limited primarily to high-risk infants who qualified for a different, monthly injection. Nirsevimab offers broad protection with a single dose, streamlining the process and making this critical safeguard accessible to a much wider population of vulnerable babies. It’s akin to providing an immediate, passive immunity that helps their tiny bodies fight off the virus, potentially preventing thousands of hospitalizations and doctor visits each year.
“This is truly a game-changer for pediatric health, offering unprecedented protection and peace of mind to countless parents during RSV season,” commented Dr. Anya Sharma, a pediatric infectious disease specialist. “It’s a clear example of how scientific innovation, when carefully evaluated, can profoundly improve public health outcomes for our most vulnerable.” The move underscores the CDC’s commitment to integrating cutting-edge science into standard care when the evidence strongly supports it.
Maintaining the High Bar: A Controversial Candidate is Denied
In stark contrast to the RSV approval, the CDC also recently turned down a proposal for a new childhood vaccine aimed at a different, though common, ailment. While the specifics of the vaccine itself remain under wraps given its rejection, sources close to the deliberations indicate it was a novel approach targeting a widespread, albeit typically mild, childhood viral infection – one that doesn’t usually pose life-threatening risks to healthy children. The proposal was seen by some as controversial, with questions raised about its necessity for universal recommendation and the long-term data available.
The CDC’s decision to decline this vaccine’s recommendation reportedly hinged on several factors, including insufficient evidence of significant public health benefit for widespread application, questions regarding its long-term efficacy and safety profile across diverse pediatric populations, and potentially its cost-effectiveness compared to the severity of the disease it aimed to prevent. The agency’s advisory committee, known for its meticulous review process, evidently found that the proposed vaccine did not meet the rigorous standards required for inclusion in the routine childhood immunization schedule.
This rejection serves as a powerful reminder of the CDC’s stringent approval process. It highlights that even with a growing array of infectious diseases, not every vaccine that enters development will ultimately earn a recommendation for broad public use. The agency consistently prioritizes vaccines that address significant public health threats, demonstrate clear efficacy and safety, and offer a strong benefit-to-risk ratio for the populations they serve. This decision, though disappointing to some proponents, reinforces the CDC’s role as a guardian of public health, ensuring that only the most impactful and thoroughly vetted interventions become standard.
Balancing Progress with Prudence
These two recent decisions by the CDC paint a clear picture: an agency dedicated to leveraging scientific advancements to protect public health, especially for children, but equally committed to an unwavering standard of evidence. Approving a vital new tool against RSV while holding the line on a controversial, less-proven candidate demonstrates a balanced approach – one that embraces innovation when justified and exercises caution when the data isn’t definitive. It reinforces the foundation of trust in public health recommendations, ensuring that every addition to a child’s health regimen is there for a reason, backed by the best available science.